Introduction
Positive End-Expiratory Pressure (PEEP) is a cornerstone in the management of patients requiring mechanical ventilation, especially in cases of Acute Respiratory Distress Syndrome (ARDS) and other forms of respiratory failure. Its ability to improve oxygenation is well-documented, but the underlying mechanisms, particularly when viewed through the lens of Fick’s Law of Diffusion, provide a deeper understanding of how PEEP functions at a physiological level. This article delves into the details of how PEEP enhances oxygenation, explaining the process through the principles of Fick’s Law, and its application to alveolar epithelial cells.
Understanding Fick’s Law of Diffusion
Fick’s Law of Diffusion is a fundamental principle that describes the rate at which a gas diffuses across a membrane. The law is mathematically represented as:
V=A×ΔPTV = \frac{A \times ΔP}{T}
Where:
- V is the rate of diffusion,
- A is the surface area available for diffusion,
- ΔP is the difference in partial pressures of the gas across the membrane,
- T is the thickness of the membrane.
This equation tells us that the rate of diffusion of a gas is directly proportional to the surface area of the membrane and the difference in partial pressures, while it is inversely proportional to the thickness of the membrane [1].
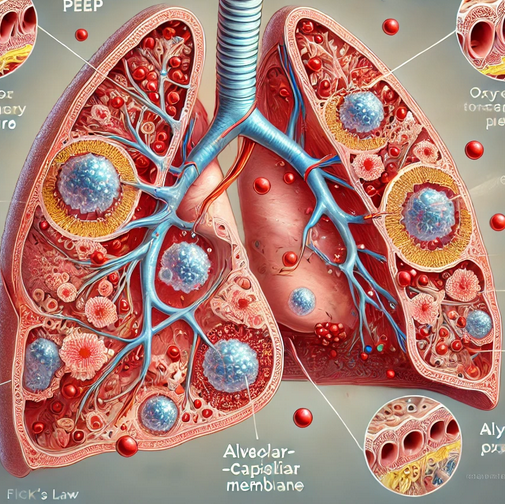
Application of Fick’s Law to the Lungs
In the context of the lungs, Fick’s Law describes how oxygen (O₂) diffuses from the alveoli into the pulmonary capillaries, while carbon dioxide (CO₂) diffuses in the opposite direction [1]. The alveolar-capillary membrane is where this gas exchange occurs, and the efficiency of this process is crucial for maintaining adequate oxygenation of the blood [1].
- Surface Area (A): The alveoli are tiny, balloon-like structures in the lungs that provide a large surface area for gas exchange. This large surface area is critical for the diffusion of oxygen into the blood. In conditions such as ARDS, where alveoli collapse or are filled with fluid, the effective surface area for gas exchange is significantly reduced, impairing oxygenation [2, 3].
- Partial Pressure Difference (ΔP): The driving force for the diffusion of gases across the alveolar-capillary membrane is the difference in partial pressures of the gases. Oxygen will diffuse from an area of higher partial pressure (the alveoli) to an area of lower partial pressure (the capillaries). In cases of hypoxemia, the partial pressure of oxygen in the alveoli is reduced, diminishing the gradient and thus the rate of oxygen diffusion [1, 4].
- Thickness of the Membrane (T): The thickness of the alveolar-capillary membrane also plays a critical role in gas exchange. Inflammation, edema, or fibrosis can thicken this membrane, making it more difficult for gases to diffuse. This increased thickness further impairs oxygenation, as per Fick’s Law [4].
How PEEP Enhances Oxygenation
PEEP works by increasing the pressure in the lungs at the end of expiration, preventing the alveoli from collapsing and recruiting alveoli that have already collapsed. The physiological effects of PEEP can be analyzed through the components of Fick’s Law:
- Increasing Surface Area (A): By preventing alveolar collapse and reopening collapsed alveoli, PEEP effectively increases the surface area available for gas exchange. In conditions like ARDS, where alveolar collapse is a significant issue, PEEP plays a critical role in maintaining the surface area necessary for adequate oxygenation [3].
- Enhancing Partial Pressure Difference (ΔP): PEEP also increases the mean airway pressure, which helps maintain or elevate the partial pressure of oxygen in the alveoli. This increase in alveolar pressure raises the ΔP in Fick’s equation, driving more oxygen across the alveolar-capillary membrane into the blood. By optimizing the partial pressure difference, PEEP ensures a more effective and efficient transfer of oxygen to the bloodstream [1, 5].
- Mitigating the Effects of Membrane Thickness (T): In conditions where the alveolar-capillary membrane is thickened, such as in pulmonary edema or fibrosis, PEEP can help by keeping the alveoli open and reducing the impact of the increased membrane thickness on gas exchange. While PEEP cannot directly reduce the thickness of the membrane, by maintaining alveolar stability, it can optimize the conditions for diffusion across the thickened membrane [4].
Clinical Implications and Considerations
The application of PEEP must be carefully tailored to each patient’s condition. While PEEP can significantly improve oxygenation, especially in ARDS, it must be balanced against potential risks, such as barotrauma, volutrauma, and hemodynamic instability [6]. The key is to find the “optimal PEEP” that maximizes oxygenation while minimizing these risks [6].
Moreover, the use of PEEP is often combined with other ventilation strategies, such as lung-protective ventilation, to ensure that the benefits of improved oxygenation are realized without causing additional lung injury [7]. Understanding the underlying principles, including Fick’s Law, allows clinicians to make informed decisions about the appropriate levels of PEEP and other ventilatory parameters.
Conclusion
PEEP is a powerful tool in the management of respiratory failure, offering significant benefits in terms of oxygenation. By applying Fick’s Law of Diffusion, we can better understand how PEEP enhances oxygenation at a cellular level, particularly in the alveoli. It increases the surface area available for gas exchange, enhances the partial pressure difference driving oxygen into the blood, and helps mitigate the adverse effects of increased membrane thickness.
In clinical practice, the thoughtful application of PEEP, guided by an understanding of these physiological principles, can lead to better patient outcomes, especially in critically ill patients with compromised lung function.
References
- West, J. B. (2012). Respiratory Physiology: The Essentials. 9th Edition. Lippincott Williams & Wilkins.
- ARDS Definition Task Force. (2012). Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA, 307(23), 2526-2533.
- Gattinoni, L., et al. (2006). Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med, 354(17), 1775-1786.
- Ware, L. B., & Matthay, M. A. (2000). The acute respiratory distress syndrome. N Engl J Med, 342(18), 1334-1349.
- Papazian, L., et al. (2016). Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care, 6(1), 51.
- Dreyfuss, D., & Saumon, G. (1998). Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med, 157(1), 294-323.
- Amato, M. B., et al. (1998). Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med, 338(6), 347-354.